Rebecca Hull-Meichle

Rebecca Hull-Meichle
Ph.D., University of Nottingham (UK)
Professor and Canada Excellence Research Chair
AWARDS
- Canada Excellence Research Chair in the Islet Microenvironment (read more about this here)
RESEARCH INTERESTS
Our group is focused on understanding how and why people develop diabetes, by investigating mechanisms leading to loss of insulin release from the pancreatic β cell. In particular, we study the microenvironment or “neighbourhood” in which the β cells live and how changes in this microenvironment contribute to diabetes development.
Theme 1: Interactions between the exocrine and endocrine pancreas
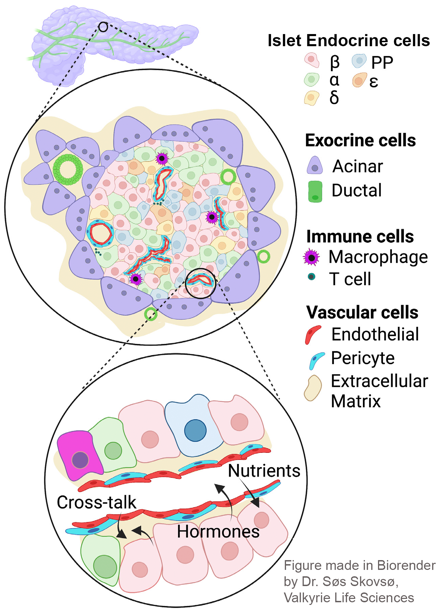
Islets exist within a vast “ocean” of the exocrine pancreas. The role of the exocrine pancreas in maintaining islet function is poorly understood, but its importance is suggested by a strong association between diseases of the exocrine pancreas and diabetes. Our work in this area is focused on cystic fibrosis-related diabetes, and understanding the importance of exocrine-endocrine pancreas interactions in its development.
Interactions between pancreatic ductal epithelial cells and other cells of the exocrine and endocrine pancreas
Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) result in cystic fibrosis and primarily affect cells with high CFTR expression. In the exocrine pancreas, ductal cells are the main CFTR-expressing cells, and their function is profoundly affected in CF. We are undertaking a series of studies using cells from human donors and stem cell-based models to understand how loss of CFTR in ductal cells may impair function of β cells along with other cell types within the islet and exocrine pancreas.
The cystic fibrosis pancreas under the microscope
The original description of cystic fibrosis almost 100 years ago was made based on microscopic evidence of pancreatic disease. However, our understanding of the nature of pathological changes in the CF pancreas remains incomplete. Our ongoing work to systematically map changes in the abundance and arrangement of cell types within the CF pancreas is an important component in clarifying the steps that lead to CFRD. Current projects include detailed mapping of the vasculature of the exocrine and endocrine pancreas which we have shown to be profoundly altered in CF.
Comprehensive analysis of the cystic fibrosis pancreas.
In parallel to the cell biology and microscopy-based studies described above, we are also conducting the first analysis of gene expression and gene regulation in the CF pancreas, using single nuclear RNAseq, ATACseq and spatial transcriptomics. The data we are generating will provide the first comprehensive, unbiased picture of gene expression within all cell types of the CF pancreas and will provide important clues to underlying mechanisms of CFRD that we will take back to study further in our cell culture systems.
Theme 2: Importance of the Islet Vasculature in Health and Disease
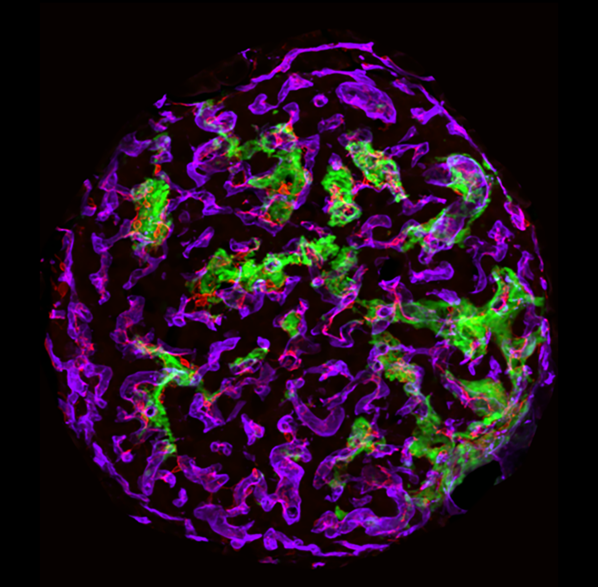
Islets have a robust vascular supply, to allow delivery of nutrients and circulating stimuli to β cells, and deliver insulin and other islet hormones through the bloodstream to their target tissues. The main cells of the islet vasculature – microvascular endothelial cells and pericytes – also provide a range of factors that maintain β cells in a optimally functioning state. Conversely, in diabetes, the islet vasculature becomes inflamed and destabilized. Our ongoing work is aimed at understanding mechanisms that promote diabetes-induced vascular destabilization in the islet, the functional significance of this destabilization on insulin release and identification of candidate molecules that might improve or prevent islet vascular destabilization in diabetes. This work includes in vitro microvascular reconstitution assays and mouse models of diabetes-related microvascular disease.
Selected Publications
- Malik SS, Padmanabhan D, Hull-Meichle, RL: Pancreas and islet morphology in cystic fibrosis: Clues to the etiology of cystic fibrosis-related diabetes. Front Endocrinol 14:1269139, 2023. DOI 3389/fendo.2023.1269139
- Mastracci TL, Apte M, Amundadottir LT, Alvarsson A, Artandi S, Bellin MD, Bernal-Mizrachi E, Caicedo A, Campbell-Thompson M, Cruz-Monserrate Z, El Ouaamari A, Gaulton KJ, Geisz A, Goodarzi MO, Hara M, Hull-Meichle RL, Kleger A, Klein AP, Kopp JL, Kulkarni RN, Muzumdar MD, Naren AP, Oakes SA, Olesen SS, Phelps EA, Powers AC, Stabler CL, Tirkes T, Whitcomb DC, Yadav D, Yong J, Zaghloul NA, Pandol SJ, Sander M: Integrated Physiology of the Exocrine and Endocrine Compartments in Pancreatic Diseases: Workshop Proceedings. Diabetes. 2023 Apr 1;72(4):433-448 and 2022 Oct 1;51(9):1061-1073. DOI 10.2337/db22-0942
- Castillo JJ, Aplin AC, Hackney DJ, Hogan MF, Esser N, Templin AT, Akter R, Kahn SE, Raleigh DP, Zraika S, Hull RL. Islet amyloid polypeptide aggregation exerts cytotoxic and proinflammatory effects on the islet vasculature in mice. Diabetologia. 2022 Oct;65(10):1687-1700. DOI 1007/s00125-022-05756-9
- Hogan MF, Hackney DJ, Aplin AC, Mundinger TO, Larmore MJ, Castillo JJ, Esser N, Zraika S, Hull RL. SGLT2-i improves markers of islet endothelial cell function in db/db diabetic mice. J Endocrinol. 2021 Feb;248(2):95-106. doi: 10.1530/JOE-20-0354.
- Hull RL, Gibson RL, McNamara S, Deutsch GH, Fligner CL, Frevert CW, Ramsey BW, Sanda S. Islet Interleukin-1β immunoreactivity is an early feature of cystic fibrosis that may contribute to β-cell failure. Diabetes Care. 2018 Apr;41(4):823-830. DOI 2337/dc17-1387
Laboratory members
Dr. Birbickram Roy (Research Associate; birbickr@ualberta.ca)
Dr. Kunimasa Suzuki (Research Associate; kunimasa@ualberta.ca)
Dr. Jocelyn Manning Fox (Program Support; jm33@ualberta.ca)