Our Facility
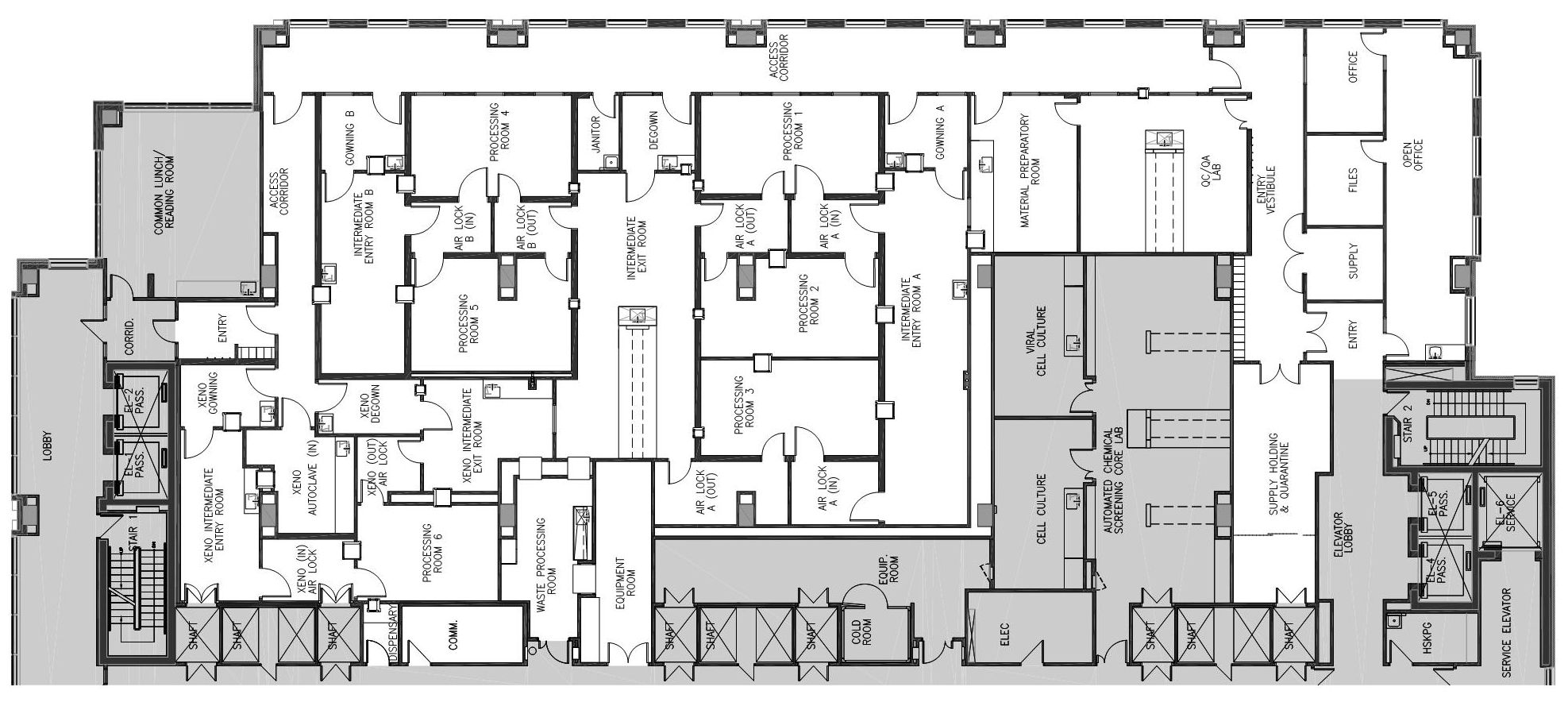
Our state-of-the-art facility occupies approximately 10,000 square feet, allowing us to be at the forefront of delivering safe, efficient, and high-quality cell therapy products to meet the ever-evolving needs of the medical community.
Our facility features six distinct GMP processing rooms meticulously designed to prevent cross-contamination, ensuring the utmost safety and purity of our cell therapy products. Each room operates independently, creating a controlled environment that adheres to stringent regulatory guidelines and international standards.
GMP Processing Rooms
Manufacturing capacity for up to 6 simultaneous products with cleanroom temperature, humidity and differential pressure monitoring.
Learn More
Quality Control Laboratory
Our dedicated Quality Control Laboratory plays a role in ensuring the safety, efficacy, and quality of all our cell therapy products.
Learn More
Support Areas
Our comprehensive support areas include Gowning Rooms, Intermediate Entry/Exit, Airlocks, Waste Processing and more.