Partners
DDIC has established a collaborative relationship with regional organizations with complementary expertise in drug development. This partnership enables seamless transition of drug candidates from lab to market.
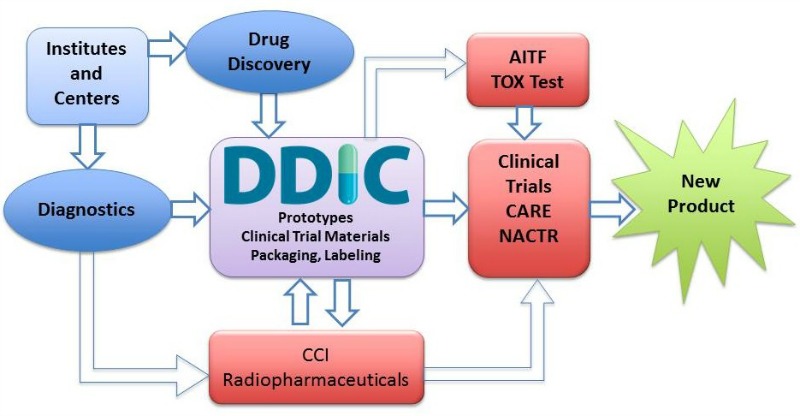
- Work begins at institutes and centers
- It can move to Drug Discovery or Diagnostics
- If moving to Drug Discovery, then it goes to the Drug Development and Innovation Center (DDIC) Prototypes Clinical Trial Materials Packaging, Labeling
- If moving to Diagnostics, it can then either go to the DDIC or Cross Cancer Institute (CCI) Radiopharmaceuticals
- If moving to CCI Radiopharmaceuticals, it can then either move back and forth to the DDIC or move on to Clinical Trials Complementary and Alternative Research and Education (CARE) Northern Alberta Clinical Trials Research Center (NACTR)
- From DDIC, it can either move to Alberta Innovates Technology Futures (AITF) TOX Test, CCI Radiopharmaceuticals, or to Clinical Trials CARE NACTR
- If moving to AITF TOX Test, it can then move to the Clinical Trials CARE NACTR
- If moving to CCI Radiopharmaceuticals, it can then either move back and forth to the DDIC or move on to Clinical Trials Complementary and Alternative Research and Education (CARE) Northern Alberta Clinical Trials Research Center (NACTR)
- From Clinical Trials CARE NACTR, it then becomes a new product.
Acronyms. DDIC: Drug Development and Innovation Center; AITF: Alberta Innovates Technology Futures; CCI: Cross Cancer Institute; NACTRC: Northern Alberta Clinical Trials Research Center; CARE: Complementary and Alternative Research and Education
DDIC has close linkages with global organizations such as United States Pharmacopoeia (USP), American Association of Pharmaceutical Scientists (AAPS), and universities and companies in South Korea, China, Brazil and Germany.
Success Stories
DDIC has successfully developed products for start-up companies, provided regulatory guidance to others and has been instrumental in development and approval of various drug products discovered by university researchers. Some of the past and present collaborators of the Centre include: Alberta Rhodiola Rosea Growers Organization (ARRGO), IgY Inc., Alberta based Pharmaceutical and NHP companies and United States Pharmacopoeia (USP).