
Ray Rajotte and James Shapiro
In the summer of 2000, a global spotlight shone momentarily on Edmonton. A small team of University of Alberta researchers had done what until then had been impossible, helping patients with Type 1 diabetes become insulin-independent at an unprecedented success rate of 100 per cent. Termed the "Edmonton Protocol," the procedure became a ray of hope for those suffering from diabetes worldwide.
Few fully understood all the setbacks that had been overcome to get to that point. Even fewer could have predicted the lows and highs yet to come. But Ray Rajotte, professor of surgery at the U of A and the founder of its Islet Transplant Group, lived through it all.
"There were probably more failures than successes," says Rajotte, reflecting back on his years working in the field of islet transplantation. "Why would I continue to work in an area for so much of my career? Some would say, 'Gee, you're pretty bullheaded and stubborn.' And I guess I was."
Steering through rough waters to lay a course forward
The foundations of the Edmonton Protocol had their beginning in the pioneering work of Ray Rajotte starting in 1972-28 years before its breakthrough success. As a U of A biomedical engineer back then, Rajotte recalls sitting in on a lecture by American researcher Paul Lacey who had cured diabetic rats by transplanting the islet cells of healthy rats into them. In that moment, he quickly saw a new path for his work in the isolation and cryopreservation of islet cells, with the hope it might one day have application in humans.
Over the next several years, Rajotte spent his time in research labs scattered across the United States learning all he could about isolating islets. In 1979, he returned to the U of A to join the departments of surgery and medicine, and began using his newfound knowledge with a goal of moving his efforts from animal to human islets. Rajotte began to piece together the team that would make up the initial Islet Transplantation Group. It consisted of himself, graduate students and surgical fellows Garth Warnock and Norman Kneteman, and endocrinologist Eddie Ryan.
"There were probably more failures than successes. Why would I continue to work in an area for so much of my career? Some would say, 'Gee, you're pretty bullheaded and stubborn.' And I guess I was." -Ray Rajotte
Isolating the insulin-producing islets posed a major challenge. If donor islets could somehow be isolated, it was postulated that they could be injected into the liver. There, the islets would become revascularized and perform their duties as usual, away from the besieged pancreas, the site where a diabetic's immune system inexplicably attacks healthy islets. With any luck, such a transplant-accompanied by a proper anti-rejection drug regimen-could allow a Type 1 diabetic to be free of insulin injections.
By the late 1980s, Rajotte's team felt it was finally ready for the next step-a clinical trial of human islets. In 1989, the group carried out Canada's first islet transplant.
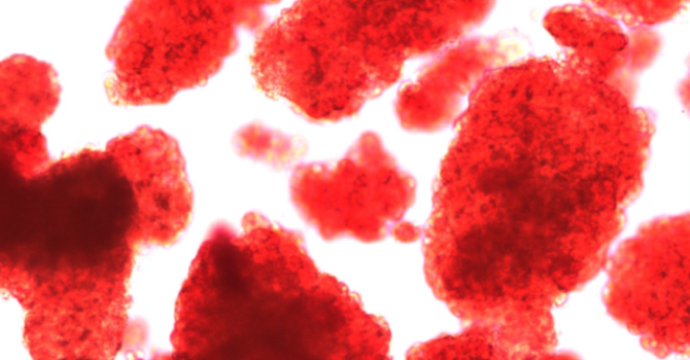
"The first patient we transplanted, his insulin requirement went down, but he didn't quite get off insulin," says Rajotte. "The same thing happened in a second patient."
Undeterred, the team set about adjusting their methods. For a third patient, they doubled the number of islets being injected, using a mixture of fresh and cryopreserved islet cells. Though not perfect, the adjustment proved increasingly successful. The patient achieved insulin independence for two and a half years.
"In all we did five islet transplants," says Norman Kneteman, now director of the Division of Transplantation Surgery at the U of A. "Amongst those five we had two patients that we managed to get off insulin for extended periods of time. Those were some of the first in the world to achieve insulin independence … but the success that we had was not prolonged. The best that we did was just over two years."
"Worldwide there were probably about 260 transplants carried out and only eight per cent of the patients got off of insulin," adds Rajotte. "Eight per cent just wasn't good enough."
They needed to become better. They needed to go back to the drawing board.
With no clear path forward, the U of A scientists returned to the lab, suspending islet transplants for the time being to focus once again on advancing the science before bringing it back to patients.
"There was a period of time where we wondered how to go forward," says Kneteman.
The team was at a standstill. For five years work continued in labs, yet the clinical program lay fallow. It needed a fresh start and reinvigorated leadership-which came with the arrival of a promising young surgeon from England.
"My contribution was leading this clinical team, but also throwing the kitchen sink at the protocol. From where I stood there was absolutely nothing to lose." -James Shapiro
Capturing the world's attention
In 1993, James Shapiro was recruited to the U of A as part of a transplantation fellowship. While in Edmonton, Shapiro also worked on his PhD, studying new anti-rejection drugs and steroid combinations for possible testing on islet transplantation. He would go on to posts in Vancouver, Japan and the University of Maryland before being recruited back to the U of A in 1998 to lead and rejuvenate what had then become a stalled Clinical Islet Transplant Program.
"I personally really didn't have a lot of hope that islet transplantation was ever going to succeed," says Shapiro, reflecting in his Edmonton office. "When the shackles of this program were passed on to me as the director, the program had laid dormant with no transplant activity in the preceding five years. The collagenase enzymes were unstable at best, and there was very little enthusiasm to drive forward a program where the global results were only a dismal eight per cent."
The team, bolstered with the key additions of Jonathan Lakey and Greg Korbutt, decided the time for small steps was in the past. There were two large modifications made to the procedure. Where the initial wave of patients had all previously had a kidney transplant as well, the team decided the next wave of patients would not. This change to the type of patient selected for the procedure gave the team complete freedom to use anti-rejection drugs of their choosing. As a result of that decision, the anti-rejection drugs used with the procedure were overhauled completely. New drugs were chosen in the hopes they would be less toxic to the islets.
"My contribution was leading this clinical team, but also throwing the kitchen sink at the protocol," says Shapiro. "From where I stood there was absolutely nothing to lose."
"Normally when you do a scientific experiment, you change one variable and you see if it has impact or not. But it seemed pointless to do that. I didn't think one variable on its own was ever going to make a difference."
"We had to take a leap of faith," remembers Kneteman.
In all there were 17 changes from the treatment offered to patients in 1989. On March 11, 1999, Bryon Best, a teacher from the Northwest Territories, became the first patient to receive the revised islet transplant. Within a week he no longer needed insulin injections and was able to maintain a steady glucose count.
Not trusting a one-off result, Shapiro's team went back to work, treating six more patients under the new protocol. It was only after they had successfully transplanted islets in seven patients that the team realized their success. All seven were free of insulin injections-something that had never happened before.

The Edmonton Protocol was born.
"I was in Chicago [presenting the results to the American Society of Transplantation] and I got a standing ovation from the entire crowd of around 5,000 transplant clinicians and scientists," Shapiro recalls.
"Here in Edmonton things went absolutely berserk. There was media here. There were patients from all over the world flooding our hospital switchboard, desperate to get onto the islet transplant list."
"I came back to Edmonton a couple of days later and walked into my office and there was a pile of letters. There must have been two or three thousand people who had written-again, the same thing, 'How can I get on the transplant list?'"
"It was a tremendously exciting time for all of us to see that big success and to see how it captured the imagination of people across the country, across the continent and, in many cases, around the world," Kneteman adds.
It was a giant step forward in the treatment of Type 1 diabetes. But it wasn't a cure.
"Here in Edmonton things went absolutely berserk. There was media here. There were patients from all over the world flooding our hospital switchboard, desperate to get onto the islet transplant list." -James Shapiro
Over time the impact of the islets faded, and one by one, within five years, most patients went back to using small amounts of insulin. By year five, only 11 per cent of patients remained insulin-free. Because of the difficulty in securing islet cells-only available from donated pancreases-the new protocol could also only be used for the most severe cases of Type 1 diabetes, severely limiting the number of patients that could be treated.
Still, it paved a way forward.
"It rejuvenated the field," says Greg Korbutt, now a professor of surgery at the U of A and a member of the Alberta Diabetes Institute. "It proved that it was possible, but it also showed that it needed to be improved."
In the years since, the collaborators have worked to do just that.
Where do we go from here? The Islet Transplantation Group today
As of September 2016, the Clinical Islet Transplant Program has treated 249 patients and performed more than 570 islet infusions. Through continuous research and modifications to the Edmonton Protocol and the techniques used, 60 per cent of patients are now insulin independent for at least five years after the procedure. Based on the team's findings, research efforts have spread around the world, with dozens of labs working to build on the success first achieved in Edmonton.
The program, which started out funded by the Alberta Diabetes Foundation from 1998 to 2000, became fully funded by Alberta Health Services in 2001 as the standard of care for Type 1 diabetes. Since then, it has also been fully funded in the United Kingdom, Italy and Switzerland. The United States is expected to soon follow suit.
"No question the islet transplant group in Edmonton remains the best in the world," says Rajotte. "I have no question, no doubt in my mind about that. We do the most transplants and we continue to have the best success."
With so much promise, the Edmonton researchers know much of it remains unfulfilled. The work, now decades old, is still far from the ultimate goal-a complete cure that is available for everyone.
In the near term, the researchers are in a race to solve two problems.
"We know islet transplantation works, but what we really want to do is develop an unlimited source of islets," says Rajotte. "One of the limitations is that there are just not enough human organs to go around. We also need to figure out ways to carry out the transplant without the need for anti-rejection drugs."
"The true future would be to be able to do transplants without needing to have the patients take lifelong immunosuppression," adds Korbutt. "That's why we're not transplanting a 12-year-old child that just got recently diagnosed with Type 1 diabetes, because the risks of the transplant and taking immunosuppression are greater than the risk of simply taking insulin."
Possible solutions are on the horizon. Korbutt and other Alberta Diabetes Institute members such as Gina Rayat, a professor of surgery at the University of Alberta, are advancing efforts to develop a safe source of islets from neonatal pigs for clinical use. Shapiro is taking a different approach. In his view, the future of the Edmonton Protocol is in the use of stem cells during the transplantation procedure rather than islet cells.

Shapiro's theory is if they were to take seed cells-precursors to islet cells-and transplant them into the body, they would become strongly producing islet cells over a period of months. He is currently working with a U.S. company named Viacyte and together they are in the midst of a clinical trial putting the theory to the test. They are also experimenting with the location of the cell transplant, moving it from the liver to a site just underneath the skin in a device that prevents the body's immune cells from coming into direct contact with the transplanted stem cells.
"The idea is that you could do this without needing any anti-rejection drugs at all. If that happened, it would be a home run," says Shapiro.
"There is always fear in the back of your mind. It is always there. Diabetes is doing its best every day to kill you, and you are doing your best to stop it from doing that. To be free of that, to not have to worry about that-even for a week-I couldn't even tell you what that would be like. It would be unbelievable." -Kerry Elliott
In August of 2015, Kerry Elliott was the first patient in Edmonton to undergo a stem cell transplant as part of the clinical trial. Originally diagnosed with Type 1 diabetes in 1982, Elliott had followed Shapiro's career for over two decades and reached out to Shapiro's team after hearing of his new efforts.
"He's just a remarkable physician who seems to hit the bullseye an awful lot," says Elliott. "Going into this there was no illusion that I would be suddenly cured and free of insulin forever. But I knew that I had the opportunity to help get the ball rolling."
The aim of the current phase of the clinical trial is simply to prove that stem cells are safe to use in humans and act as the researchers expect them to. Elliott had a small amount of stem cells contained within plastic, semi-porous pods implanted in his abdomen and under his skin. The amount of cells given were not expected to be enough to produce as much insulin as a person would need. According to Elliott, the expectation hit the mark, but the infusion has still made a difference by cutting the long-lasting insulin he requires each day in half.
If the current phase of the clinical trial proves both safe and successful, future steps will involve testing stem cells as a possible treatment. Eventually Shapiro believes the stem cell approach could evolve into a form of personalized medicine, where doctors use a patient's own stem cells for a transplant that would be completely compatible with their immune system, eliminating the need for anti-rejection drugs.
Elliott believes Shapiro's team is on the cusp of yet another breakthrough-one that would be life-changing for people like him.
"There is always fear in the back of your mind. It is always there. Diabetes is doing its best every day to kill you, and you are doing your best to stop it from doing that," says Elliott. "To be free of that, to not have to worry about that-even for a week-I couldn't even tell you what that would be like. It would be unbelievable."
While the path to a Type 1 diabetes cure will still be filled with bumps, potholes and detours along the way, the U of A's Clinical Islet Transplant Program continues to forge the way forward. And though they are not quite there yet, they know their ultimate goal is closer than ever to being fulfilled.
"I hope within my lifetime the Edmonton Protocol can become the treatment of choice for all people with diabetes," says Rajotte.
"It's not where we are today but where we're going next that's most exciting," adds Shapiro. "It's important to have all the best approaches running in parallel, to maximize the chance that one ultimate breakthrough will finally reach the finish line. The solution is just within our grasp."