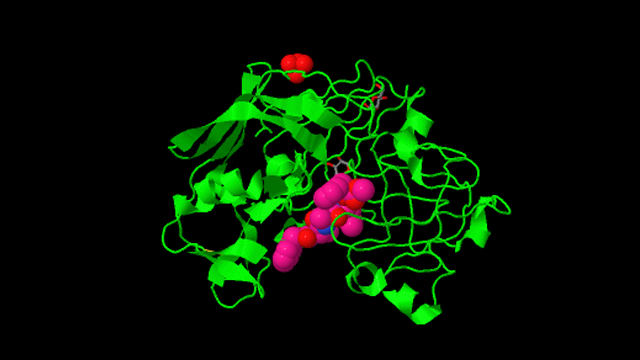
Structure of penicillopepsin.
The University of Alberta's Department of Biochemistry has a long history of research firsts, beginning with James Collip's work in 1924 on the parathyroid hormone, which regulates calcium levels in the body.
1974: First 3-D structure of a protein in Canada. Michael James determined the structure of Streptomyces griseus Protease B (SGPB) in collaboration with colleague Larry Smillie, who had determined the linear amino-acid sequence of the enzyme.
1974: The Medical Research Council (MRC) Group in Protein Structure and Function was formed with Cyril Kay and Smillie as directors.
1975: Brian Sykes, Bob Fletterick and Bob Hodges joined the U of A Department of Biochemistry and MRC Research Group. Sykes became the leader in the development of biological nuclear magnetic resonance in Canada.
Over the period of 1974-1995, the Group published 1,600 publications in top international biochemical journals and trained more than 200 graduate students and postdoctoral fellows.
The labs of Sykes, Smillie, Kay, James and Hodges made unique contributions to our understanding of the chemistry and structure of both muscle and non-muscle systems, and the molecular mechanism by which calcium triggers muscle contraction in normal and diseased states.
1976: First 3-D structure of an aspartic protease. The structure of penicillopepsin was determined in James' lab by I-Nan Hsu and Louis Delbaere, in collaboration with Theo Hofmann at the University of Toronto. This work formed the basis for future research on renin, pepsin, and the retroviral aspartic peptidase, HIV protease, an enzyme that is critical for the replication and life cycle of the HIV virus.
1978: Fletterick, in collaboration with Neil Madsen, established the three-dimensional structure of phosphorylase a, thereby providing a structural basis for understanding the regulation of its activity by various effectors.
1981:Wayne Anderson determined the first high-resolution structure of the Cro repressor protein, a DNA-binding protein, providing weight into the mechanism by which proteins regulate gene expression.
1985: First 3-D structure of Troponin C (TnC), the calcium-binding protein in muscle, and the first atomic resolution of any muscle contraction protein. James determined the structure of TnC in collaboration with Smillie who determined its sequence, and Kay who determined its size and shape.
1989: First 3-D structure of Renin. James' work led to the development of drugs to control high blood pressure.
1990: Kay, Hodges and Sykes became founding members of the Protein Engineering Network of Centers of Excellence (PENCE) with a mandate to engineer and study proteins for the economic benefit of Canada.
1990:Charles Holmes and Zygmunt Derewenda joined the MRC Group.
1990-2004:Hodges, in collaboration with Kay, chemically synthesized polyamino acid models of proteins to yield important information about the forces stabilizing alpha-helix and beta conformations, coiled coils and metal binding proteins.
1992: First structure of TEM-1, the -lactamase enzyme that makes E.coli penicillin-resistant. Postdoctoral fellow Natalie Strynadka, '83 BSc, '90 PhD, determined the structure of TEM-I while working in James' lab. This structure contributes to our understanding of antibiotic-resistant bacteria.
1994: First structure of the protein inhibitor BLIP, the -lactamase inhibitor protein. Strynadka determined the structure of the protein BLIP, a potent inhibitor of the -lactamase enzyme that makes bacteria antibiotic resistant.
1996:Sykes became the Director of the Canadian National High Field NMR Facility (NANUC) based in Edmonton.
2000:Holmes demonstrated the molecular mechanism underlying marine toxin regulation of signal transduction.
2000-2003:Sykes, in collaboration with Ian Clark-Lewis, University of British Columbia, determined the NMR solution structure of several chemokines.
2013 to present: First 3-D structure of enzyme ⍺-L-iduronidase. James' latest research focuses on alternative treatments for mucopolysaccharidosis type 1. Currently, patients are treated with enzyme-replacement therapy, which requires them to be injected every two weeks, and is often prohibitively costly. James is helping to develop small molecules that will bind to the enzyme to help it fold so that it can travel to the lysosomes, where it needs to be, undetected by the immune system.