MMDx-Kidney System
Investigators
Drs. Philip F Halloran, Konrad Famulski, Jeff Reeve, and the MMDx-Kidney Study Group (ClinicalTrials.gov NCT01299168)
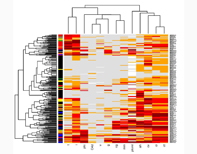
Our kidney program uses microarray analysis of transcriptional changes as a tool to improve diagnosis and to understand the pathogenesis of various human diseases including allograft rejection.
A major focus has been to define the markers that differentiate T cell-mediated rejection (TCMR) from antibody-mediated rejection (ABMR) - please see The ATAGC histology regression equations and Reference Standard classification for kidney transplant biopsies. This is significant because these events require different treatment strategies, resulting in different treatment responses and graft outcomes.
Using the knowledge we generate with microarrays in concert with the clinical information and outcomes we obtain by tracking kidney transplant patients over time, we can reclassify the information in the biopsy, and improve diagnostic accuracy. We are establishing a new diagnostic system for defining the disease states in kidney transplants in order to understand, prevent, and treat the diseases that cause graft failure - please see the Molecular Microscope® System (MMDx).
MMDx was applied to a multicentre observational clinical trial in the INTERCOM trial (ClinicalTrials.gov NCT01299168), where validation of the MMDx was demonstrated.
We are currently initiating a validation study combining the molecular and histological features of transplant biopsies, plus clinical and laboratory parameters, to establish the first Integrated Diagnostic System through the INTERCOMEX study. This prospective study will include 500 unselected biopsies for clinical indications from American, Canadian, and European centers. In addition to demonstrating the feasibility and value of the MMDx in routine patient care and clinical trials, the study will develop and optimize a transparent, user-friendly reporting format to communicate this information to customers, and obtain detailed feedback from clinicians caring for the study patients. The INTERCOMEX project will further the clinical relevance of this technology by providing 24 hour turn-around time, measuring response to therapy, and providing an integrated report for each biopsy. The results will impact patient management and the design of clinical trials for the development of new drugs. Moreover, the kidney transplant MMDx system is the prototype for other organ transplants and primary organ diseases, and a Rosetta stone for reclassifying many disease states.
MMDx-Kidney Publications
- Halloran PF, Akalin E, Aubert O, Bohmig G, Brennan D, Bromberg J, et al. Real time central assessment of kidney transplant indication biopsies by microarrays: the INTERCOMEX Study. Am J Transplant 2017;17(11):2851-62.
- Reeve J, Sellares J, Mengel M, Sis B, Skene A, Hidalgo L, et al. Molecular diagnosis of T cell-mediated rejection in human kidney transplant biopsies. Am J Transplant 2013;13(3):645-55.
- Venner JM, Famulski KS, Reeve J, Chang J, Halloran PF. Relationships among injury, fibrosis, and time in human kidney transplants. Journal of Clinical Investigation Insight 2016;1(1):e85323-doi:10.1172/jci.insight.85323.
- Famulski KS, de Freitas DG, Kreepala C, Chang J, Sellares J, Sis B, et al. Molecular phenotypes of acute kidney injury in human kidney transplants. JASN 2012 May;23(5):948-58.
- Venner JM, Famulski K, Badr D, Hidalgo LG, Chang J, Halloran PF. Molecular landscape of T cell-mediated rejection in human kidney transplants: Prominence of CTLA4 and PD ligands. Am J Transplant 2014;14(11):2565-76.
- Venner JM, Hidalgo LG, Famulski KS, Chang J, Halloran PF. The molecular landscape of antibody-mediated kidney transplant rejection: Evidence for NK involvement through CD16a Fc receptors. Am J Transplant 2015;15(5):1336-48.
- Halloran PF, Venner JM, Famulski KS. Comprehensive analysis of transcript changes associated with allograft rejection: Combining universal and selective features. Am J Transplant 2017;17(7):1754-69.
- Halloran PF, Merino LM, Barreto PA. Identifying Subphenotypes of Antibody-Mediated Rejection in Kidney Transplants. Am J Transplant 2016 Mar;16(3):908-20.
- Halloran PF, Venner JM, Madill-Thomsen K, Einecke G, Parkes MD, Hidalgo LG, et al. Review: The transcripts associated with organ allograft rejection. Am J Transplant 2018;18(4):785-95.
- Madill-Thomsen KS, Wiggins RC, Eskandary F, Bohmig GA, Halloran PF. The effect of cortex/medulla proportions on molecular diagnoses in kidney transplant biopsies: rejection and injury can be assessed in medulla. Am J Transplant 2017 Feb 22;17(8):2117-28.
- Einecke G, Reeve J, Halloran PF. Hyalinosis lesions in renal transplant biopsies: time-dependent complexity of interpretation. Am J Transplant 2017;17(5):1346-57.
- Einecke G, Reeve J, Halloran PF. A molecular biopsy test based on arteriolar under-hyalinosis reflects increased probability of rejection related to under-immunosuppression. Am J Transplant 2017;18(4):821-31.
- Reeve J, Bohmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, et al. Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2017;2(12).
- Halloran PF, Böhmig GA, Bromberg J, Einecke G, Eskandary FA, Gupta G, et al. Archetypal Analysis of Injury in Kidney Transplant Biopsies Identifies Two Classes of Early AKI. Frontiers in Medicine. 2022;9(Article 817324):1-12.
- Halloran PF, Madill-Thomsen KS, Pon S, Sikosana MLN, Bohmig GA, Bromberg J, et al. Molecular diagnosis of ABMR with or without donor-specific antibody in kidney transplant biopsies: differences in timing and intensity but similar mechanisms and outcomes. Am J Transplant. 2022; 22(8):1976-1991.
- Halloran PF, Madill-Thomsen KS, Bohmig GA, Myslak M, Gupta G, Kumar D, et al. A 2-fold Approach to Polyoma Virus (BK) Nephropathy in Kidney Transplants: Distinguishing Direct Virus Effects From Cognate T Cell-mediated Inflammation. Transplantation. 2021;105(11):2374-84. 16. Madill-Thomsen KS, Bohmig GA, Bromberg J, Einecke G, Eskandary F, Gupta G, et al. Relating molecular T cell-mediated rejection activity in kidney transplant biopsies to time and to histologic tubulitis and atrophy-fibrosis. Transplantation 2023; 107(5):1102-1114.