Joanne Lemieux

Professor
Ph.D, New York University (2002)
M.Sc., Dalhousie University (1994)
Office: 780-492-3586
Lab: 780-492-3465
Fax: 780-492-0886
mlemieux@ualberta.ca
Recruiting:
The Lemieux group is currently recruiting.
Those interested in Graduate or Postdoctoral research opportunities, please send a CV and transcript to: mlemieux@ualberta.ca.
Research:
Our research centers around studying proteins involved in disease, using X-ray crystallography and other biophysical techniques, to reveal structural, functional and mechanistic details of proteins that could one day aid in the development of new drugs and vaccines.
One major focus of the lab is the study of viral proteases, such as the main protease of SARS-CoV-2, and related viruses. Peptidomimetic inhibitors have been developed that specifically inhibit the protease in vitro and in cellular assays. We aim to develop highly potent inhibitors for clinical use.Publications related to this research topic
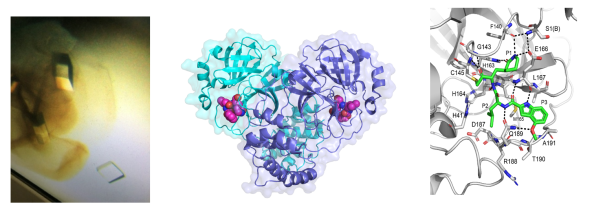
We also study membrane embedded enzymes, including the rhomboid intramembrane proteases (peptidases). Rhomboid function has been linked to Parkinson's disease, breast cancer and parasitic invasion. Our crystal structures of this novel type of protease are providing information on how the rhomboid protease acts to cleave protein targets in the membrane. Using both functional and structural approaches we are focusing on how rhomboid proteases regulate cleavage of their substrates. This information is crucial to assist with de novo drug design.
Targeted immunotherapies, such as those targeting the membrane receptors that regulate Immunecheckpoints, have revolutionized cancer therapy. Clinically these have been in the form of monoclonal antibodies, that include Ipilimumab, which targets the T-cell receptor CTLA4. However, these therapies can also lead to an immunocompromised state. We are investigating small molecule Immunecheckpoint inhibitors to develop improved therapies.
Other research topics in the lab include membrane transporters such as SERCA and Gluts.

from Lemieux et al., PNAS 2003
Complete List of Published Work in MyBibliography
Selected Publications:
Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication.
Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, Saffran HA, McKay RT, van Belkum MJ, Joyce MA, Young HS, Tyrrell DL, Vederas JC, Lemieux MJ.
Nat Commun. 2020 Aug 27;11(1):4282. doi: 10.1038/s41467-020-18096-2.
N-Terminal Finger Stabilizes the S1 Pocket for the Reversible Feline Drug GC376 in the SARS-CoV-2 M pro Dimer.
Arutyunova E, Khan MB, Fischer C, Lu J, Lamer T, Vuong W, van Belkum MJ, McKay RT, Tyrrell DL, Vederas JC, Young HS, Lemieux MJ.
J Mol Biol. 2021 Jun 25;433(13):167003. doi: 10.1016/j.jmb.2021.167003. Epub 2021 Apr 22.
Improved SARS-CoV-2 M pro inhibitors based on feline antiviral drug GC376: Structural enhancements, increased solubility, and micellar studies.
Vuong W, Fischer C, Khan MB, van Belkum MJ, Lamer T, Willoughby KD, Lu J, Arutyunova E, Joyce MA, Saffran HA, Shields JA, Young HS, Nieman JA, Tyrrell DL, Lemieux MJ, Vederas JC.
Eur J Med Chem. 2021 Oct 15;222:113584. doi: 10.1016/j.ejmech.2021.113584. Epub 2021 May 30.
Peptidomimetic α-Acyloxymethylketone Warheads with Six-Membered Lactam P1 Glutamine Mimic: SARS-CoV-2 3CL Protease Inhibition, Coronavirus Antiviral Activity, and in Vitro Biological Stability.
Bai B, Belovodskiy A, Hena M, Kandadai AS, Joyce MA, Saffran HA, Shields JA, Khan MB, Arutyunova E, Lu J, Bajwa SK, Hockman D, Fischer C, Lamer T, Vuong W, van Belkum MJ, Gu Z, Lin F, Du Y, Xu J, Rahim M, Young HS, Vederas JC, Tyrrell DL, Lemieux MJ, Nieman JA.
J Med Chem. 2021 Jul 9: acs.jmedchem.1c00616. doi: 10.1021/acs.jmedchem.1c00616. Online ahead of print.
Peptidomimetic nitrile warheads as SARS-CoV-2 3CL protease inhibitors.
Bai, B., Arutyunova, E, Khan MB, Lu J , Joyce MA, H.A., S.Saffran HA, Shields JA, Kandadai AS, Belovodskiy A, Hena M, Vuong W, Lamer T, Young HS, Vederas JC, Tyrrell DL, Lemieux MJ, and Nieman JA.
RCS Med Chem. 2021 , In press. doi: 10.1039/D1MD00247C
Insights into the catalytic properties of the mitochondrial rhomboid protease PARL.
Lysyk L, Brassard R, Arutyunova E, Siebert V, Jiang Z, Takyi E, Morrison M, Young HS, Lemberg MK, O'Donoghue AJ, Lemieux MJ.
J Biol Chem. 2021 Jan-Jun;296:100383. doi: 10.1016/j.jbc.2021.100383. Epub 2021 Feb 6.
Lab Members
Research Associate
Elena Arutyunova
Postdoctoral Fellow
Pu Chen
Fraser Ferens
Graduate Students
Raelynn Brassard
Sizhu Chen
Jimmy Lu
Cassidy Maplethorpe
Links
Location
Office: 453A MSB
Lab: 451 MSB